how to calculate concentration of solution in polarimeter|polarimetry experiment formula : Brand manufacturer The value of the optical rotation must be corrected for concentration. Figure \(\PageIndex{2}\): The effect of path length on optical rotation. The longer the path of light through a solution of . Consultar faturas. Tenha uma forma fácil e rápida para consultar e gerar seu boleto. Assim terá todas as suas faturas em aberto com a MAXX. Acesse agora e retire a 2ª via da .
{plog:ftitle_list}
Estatísticas da Mega-Sena atualizadas até o sorteio: 2692 - Dia: 24/02. Os cinco números que mais saíram e os cinco números que menos saíram na Mega-Sena. Vezes .
The plane of polarization can be determined by an instrument called a polarimeter (Figure \(\PageIndex{1}\)). Monochromatic (single wavelength) light, is polarized by a fixed polarizer next to the light source.Procedure / Determining Concentration 1. Prepare 10 mL samples of 10%, 20%, and 30% solutions of D-Glucose in water. 2. Fill the cell with 10ml of distilled deionized water and place . Generally the following equation is used to calculate the specific .The value of the optical rotation must be corrected for concentration. Figure \(\PageIndex{2}\): The effect of path length on optical rotation. The longer the path of light through a solution of .
The concentration and the optical rotation of the optically active substances in the solution are in proportion to each other. When the concentration of a sample is known, polarimetry can be applied to determine its specific rotation .
Calculate the specific rotation value using the formula: Specific Rotation = Observed Rotation / (Concentration × Cell Length) Where: Observed Rotation: The reading obtained from the polarimeter. Concentration: The concentration .Using your own sucrose sample, pour the liquid into the polarimeter cell making the pathlength as close to 10.0 cm as possible. Record the exact pathlength. Go through the same process .
The measured rotation can be used to calculate the value of solution concentrations; especially substances such as sugars, peptides and volatile oils. A polarimeter .If you know that a solution has been made from sucrose, but you don't know the concentration of the sucrose, you can measure the observed rotation, a, and calculate the concentration of .
polarimetry test formula
The specific rotation of a molecule is the rotation in degrees observed upon passing polarized light through a path length of 1 decimetre (dm) at a concentration of 1 g/mL. To convert an observed rotation to specific .A polarimeter [1] is a scientific instrument used to measure optical rotation: . Now the polarimeter tube is filled with a sugar solution of known concentration and again the analyzer is adjusted in such a way that again the equally dark point is .If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
Use the terms concentrated and dilute to describe the relative concentration of a solution. Calculate the molarity of a solution. Calculate percentage concentration (m/m, v/v, m/v). Describe a solution whose .
The procedure for preparing a solution of known concentration from a stock solution is shown in Figure \(\PageIndex{3}\). It requires calculating the number of moles of solute desired in the final volume of the more dilute solution and then calculating the volume of the stock solution that contains this amount of solute.
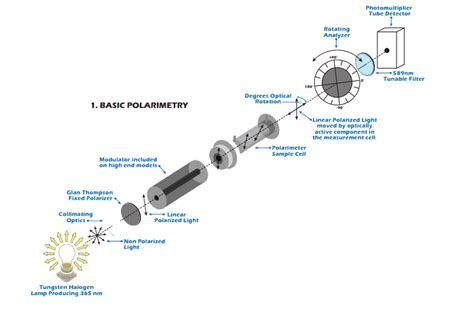
The plane of polarization can be determined by an instrument called a polarimeter, . α might be –90º or +270º rather than +90º. If the sample concentration is reduced by 10%, then the positive rotation would change to +81º (or +243º) while the negative rotation would change to –81º, and the correct α would be identified . To calculate the concentration of a solution, start by converting the solute, or the substance being dissolved, into grams. If you're converting from milliliters, you may need to look up the solute's density and then multiply that by the volume to convert to grams. Next, convert the solvent to liters.Calculate the specific rotation value using the formula: Specific Rotation = Observed Rotation / (Concentration × Cell Length) Where: Observed Rotation: The reading obtained from the polarimeter. Concentration: The concentration of the sample solution. Cell Length: The length of the polarimeter cell. By following these steps, one can use a .Solutions of solids will obviously show an effect that depends on the concentration of active material and to a small extent, both on temperature and the solvent. The Formula of Optical Rotation Optical activity is the ability of a compound to rotate the plane of polarized light.
Study Notes. A polarizer is a device through which only light waves oscillating in a single plane may pass. A polarimeter is an instrument used to determine the angle through which plane-polarized light has been rotated by a given sample. You will have the opportunity to use a polarimeter in the laboratory component of the course. An analyzer is the component of a .A polarimeter is a device that measures the rotation of linearly polarized light by an optically active sample. This is of interest to organic chemists because it enables differentiation between optically active stereoisomers, i.e., enantiomers. Enantiomers, chiral molecules, are molecules which lack an internal plane of symmetry and have a non-superimosable mirror image. One .Fig. 5.4.4 shows that five steps of logarithmic dilution on a 10% initial solution results in a concentration of 10 ppm in the final solution. Figure \(\PageIndex{4}\): Logarithmic dilution: five steps of logarithmic dilution on a 10% initial solution .If the quantity of the solute is given in mass units, you must convert mass units to mole units before using the definition of molarity to calculate concentration. For example, what is the molar concentration of a solution of 22.4 g of HCl dissolved in 1.56 L?
A solution can be described in a qualitative way by using the words concentrated and dilute. Concentrated refers to a solution with a higher amount of solute, while a diluted solution has a smaller amount of dissolved substance. If you know the concentration of a solution and dilute it, you can use the solution dilution calculator to calculate the . How to Calculate Molality of a Solution . Molality is used to express the concentration of a solution when you are performing experiments that involve temperature changes or are working with colligative properties. Note that with aqueous solutions at room temperature, the density of water is approximately 1 kg/L, so M and m are nearly the same. To calculate specific rotation, we use a device called a polarimeter, which allows us to find the rotation of plane-polarized light. This calculation is used in the specific rotation equation to .Study Notes. A polarizer is a device through which only light waves oscillating in a single plane may pass. A polarimeter is an instrument used to determine the angle through which plane-polarized light has been rotated by a given sample. .
From it you will calculate the concentration of the substance C. For example, you are taking 40 gm of cane sugar that is dissolved in water to make 100 c.c. of solution. The concentration C will be 40/100 =0.40 g/c.c. . specific rotation of sugar solution by polarimeter formula or you can say specific rotation of sugar solution by polarimeter;
Figure \(\PageIndex{1}\): The effect of concentration on optical rotation. The more concentrated the sample (the more molecules per unit volume), the more molecules will be encountered. Concentrated solutions and neat samples will have higher optical rotations than dilute solutions.
Students use a polarimeter to determine the unknown concentration of a sucrose solution. Grade Level: College. Subject: Chemistry. Student Files. Sugar Concentration through Polarimetry: 477.29 KB: . Polarimeter. Measure the concentration of optically active compounds by determining the optical rotation of their solution. Saccharimeter: This type of polarimeter is used to measure the concentration of sugar in a solution. It is commonly used in the food and beverage industry. Automatic Polarimeter: This type of polarimeter uses a digital display to measure the degree of polarization. It is commonly used in pharmaceuticals and chemistry. Given: volume of sample, solute concentration, and density of solution. Asked for: molarity of solute and mass of solute in 250 mL. Strategy: Use the concentration of the solute in parts per million to calculate the molarity. Use the concentration of the solute in parts per million to calculate the mass of the solute in the specified volume of .
This molarity calculator is a tool for converting the mass concentration of any solution to molar concentration (or recalculating grams per ml to moles). You can also calculate the mass of a substance needed to achieve a desired molarity. This article will provide you with the molarity definition and the molarity formula.. To understand the topic as a whole, .Figure \(\PageIndex{1}\): The effect of concentration on optical rotation. The more concentrated the sample (the more molecules per unit volume), the more molecules will be encountered. Concentrated solutions and neat samples will have higher optical rotations than dilute solutions.
Prepare 200 mL of a 30% sucrose solution. Fill the polarimeter tube with the sucrose solution such that the path length increases by 0.5 dm in length for each observation. Record the angles of minimum light intensity. These will be the angles of optical rotation. Prepare a graph of angle of optical rotation vs. length. The main objective of this concentration calculator is determining how to dilute a stock solution. Imagine you have a concentrated solution of hydrochloric acid. You can use this calculator to determine how much of it you need if you want to obtain 200 mL of a diluted solution with a concentration of 20 mM.The other way of enhancing this interaction is the direct change of the concentration of the chiral compound. As expected, the higher the concentration, the greater the rotation. And this as well, when doubled, the observed rotation is twice as greater. . Multiple-Choice Quiz with a 3-hour Video Solution covering the most important concepts .The polarimeter is an instrument that measures the direction and angles of rotation of plane-polarized light. The plane-polarized light passes through the sample tube containing the solution of a sample, and the angle of rotations will be received and recorded by the analyzer, as summarized in Fig. 5.4c.. Figure 5.4c Measurement of Optical Rotation with Polarimeter
two bottle choice test protocol

two bottle preference test diagram
Watch Medium — Season 1, Episode 14 with a subscription on Amazon Prime Video, Paramount Plus, or buy it on Vudu, Amazon Prime Video, Apple TV. Allison is in a .
how to calculate concentration of solution in polarimeter|polarimetry experiment formula